CapitalBio determines respiratory pathogen to aid timely and accurate diagnosis
2017-07-28
In March 2016, the Affiliated Hospital of Inner Mongolia Medical University issued a critically ill notice to a two-year-old pneumonia patient. For a young child, pneumonia can be a fatal illness.
The child had coughed for a long time and was admitted to the hospital for pneumonia. After two days of treatment, however, there was no sign of recovery. The child was experiencing severer symptoms like breathing difficulty, and was relying on a respirator to sustain life.
Under such critical circumstances, a unique and state-of-the-art detection machine was taken to the hospital, and doctors were able to use it to find the causative agent of the child's illness in little over an hour. They were then able to prescribe the correct medicine to put the patient out of danger.
The detection machine that helped determine the causative agent in such a short period of time was the respiratory pathogen detection kit produced by CapitalBio Corporation, Tsinghua University, and Peking University People's Hospital.
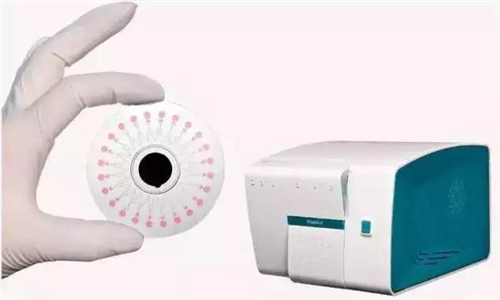
The detection kit uses a combination of microfluidics and nucleic acid amplification technologies for the first time in China, and can rapidly test for the presence of 13 types of common respiratory bacterial pathogens directly in respiratory secretions or aspirated samples, with no need for time-consuming bacterial culture. The respiratory bacterial pathogen detection kit greatly reduces the time it takes to make clinical diagnoses, improves the accuracy of the diagnosis, and hence facilitates the appropriate treatment and avoids problems associated with some bacteria that are difficult to culture.Thus far, the kit has been put into use in more than 100 hospitals in over 30 provinces, cities, and autonomous regions across China. It has succeeded in saving many lives impacted by respiratory tract infection (RTI).
RTIs are the most common infectious diseases, including tonsillitis, pneumonia, trachitis, and bronchitis.
According to a survey released by the World Health Organization in 2014, deaths caused by RTI ranked very high in low-income countries. Even in developed countries with advanced medical facilities, the number still accounted for more than 10 percent of the total number of disease-caused deaths.
Presently, RTI is most commonly treated with antibiotics, including penicillin, erythrocin, and cephalosporin. Due to frequent usage over many years, more than 10,000 antibiotics have been developed.
The widespread availability of treatment begs the question: How come respiratory infectious diseases cause such a high death toll? The answer: bacteria detection after infection.

Usually, a doctor needs to first determine, from many possible types, the specific type of bacteria that led to a patient's respiratory infection, and then choose the correct medicine for treatment. However, traditional medical detection methods take a long time, ranging from 48 hours to a month, to culture bacteria.
This makes it extremely difficult for doctors to accurately determine the infectious bacteria at the early stage of infection, resulting in the need to give prescriptions based on experience. Sometimes, this can lead to antibiotics abuse or antimicrobial resistance. When patients cannot receive a timely, accurate prescription, they are put at greater risks due to extended progression of the disease and delayed treatment.
With these things in consideration, CapitalBio Corporation and experts from Peking University People's Hospital, since 2007, have wanted to develop a detection method that makes it possible for doctors to find the pathogenic bacteria in a fast and precise manner.
In 2009, CapitalBio started R&D on the detection method. "They faced the challenge of finding the exact bacteria out of a dozen possible kinds of pathogenic bacteria within only two hours. This was an unprecedented feat in China at the time," said Wang Lei, head of the CapitalBio Corporation Biological Engineering Transformation Research Institute.
CapitalBio Corporation took five years to make breakthroughs in some key techniques, including the micro-fluidic chip, high-sensitive fluorescence detection, and quick gene amplification. In 2013, after repeated experiments and exploration, CapitalBio's team succeeded in developing the high-sensitive, multi-index respiratory bacterial pathogens caliper disc-type chip and supporting kit that shortens the detection reporting time to within two hours.
Between 2014 and 2016, CapitalBio Corporation completed its clinical trials and received China's medical equipment certificate from the administration.
CapitalBio Corporation is able to produce a caliper disc-type chip every five seconds and has produced and sold hundreds of thousands of chips to the market.
The success of the chip has brought hope to the entire micro fluidics industry. Microfluidics technology has been widely studied in the academic field, but it is little known in the field of industry application for two reasons. Firstly, nobody had found an advantageous use for the technology; secondly, large-scale production of the technology with high-precision and low-cost was difficult to achieve.
The success of CapitaBio's respiratory pathogen detection kit blazed a trail for micro-fluidic technology from lab to the market.

Why was CapitalBio Corporation able to overcome the obstacles?
"Since the beginning, CapitalBio Corporation has been established on significant advantages and foundational research. Thus, we have a full-fledged team, made of professionals in biology, gene detection, microfluidics, and scientific apparatus and instruments. It's a multidisciplinary team that really values the research and development of core technologies," said Wang.
In addition, CapitalBio has been keeping a close relationship with Tsinghua University, from where highly erudite professors power the company's technological advancement.
The new-generation microfluidic molecular diagnosis platform has helped CapitalBio work out some other series of diagnostic chips and detection kits for bacteria resistance, respiratory virus detection, digestive tract bacteria detection, and bacteria detection for wound infections.
The company has also developed a portable bacteria detection device for convenient usage in special conditions.

 Facebook
Facebook WeiXin
WeiXin CONTACT US
CONTACT US










 Tsinghua Holdings works hard for better ecological environment
Tsinghua Holdings works hard for better ecological environment