Health officials from BRI countries visit CapitalBio Technology
2018-08-22

Members of the training class take a group photo in the headquarters of CapitalBio Technology on Aug 15.
A training class consisted of health officials and medical professionals focused on tuberculosis prevention and cure from more than ten Belt and Road countries paid a visit to CapitalBio Technology, an industry-leading life science company under Tsinghua Holdings on Aug 15.
The class was organized by the International Exchange and Cooperation Center of China's National Health Commission to deepen the ties among China and BRI countries in the health sector, especially the prevention and treatment of tuberculosis, which has long been a priority for the Chinese government.
The visitors were impressed by the CapitalBio Technology's independently-developed biochip platform, a series of molecular diagnosis products and solutions for the urgent clinical needs concerning the detection of gene mutations, pathogenic microorganism and drug resistance.
They gave their thumbs up for the company's outstanding achievements in the prevention, diagnosis and cure of tuberculosis.
According to the National Health Commission, the prevention and control of tuberculosis is part and parcel to China's goal of building a moderately prosperous society and the Healthy China 2030 Initiative.
Chinese institutes and companies, like CapitalBio Technology, have shared the world with its experience and wisdom in tuberculosis control at many international conferences, including the World Health Assembly and the BRICS Health Ministers Meeting. Tuberculosis control is also of great significance to China's Belt and Road Initiative Disease Control and Prevention project.
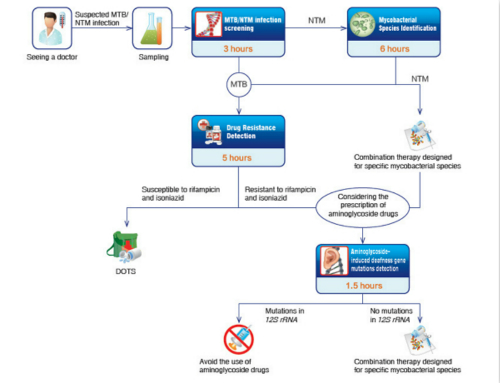
The turn-key solution for the molecular diagnosis of tuberculosis developed by CapitalBio Technology

CapitalBio Tuberculosis and Non-Tuberculous Mycobacteria Real-Time PCR Detection Kit is designed for a rapid and accurate screening of MTB/NTM infection. It employs duplex real-time fluorescence PCR technology and detects both MTB infection and NTM infection with only one test. Its detection limit for MTB is 10 CFU/PCR and its detection limit for NTM is 100 CFU/PCR. The testing process takes only 3 hours.

CapitalBio DNA Detection Kit for Mycobacterium is designed for a rapid and accurate screening of high-risk/low-risk mycobacterium tuberculosis infection. Covering 17 types of mycobacteria, they can be used to distinguish diseases (with similar symptoms) caused by the infection of different mycobacteria, help doctors make plan for personalized treatment, and evaluate the risk of postoperative recidivation.

CapitalBio Genotype and Drug Resistance Detection Kit for Mycobacterium Tuberculosis designed for a rapid and accurate genotyping of subtypes of HBV and detection of drug resistance to four drugs and interferon. It employs multiplex asymmetric allele-specific PCR technology and covers 7 drug-resistance-related mutations. It helps to ensure correct diagnosis and effective treatment.
Resorting to a state-of-the-art biochip platform, CapitalBio has been a pioneer in tuberculosis control and developed the fast molecular diagnosis system, China's first turn-key solution covering every sector of tuberculosis control, including but not limited to detection, identification and chemotherapy.
Registered in 2012 as a core subsidiary of CapitalBio Corporation and the Biological Chip Beijing National Engineering Research Center, CapitalBio Technology has developed into an industry-leading life science company with more than 500 employees, 86 percent of whom are with at least with bachelor's degrees and 14 percent are doctors or post-doctorates.

 Facebook
Facebook WeiXin
WeiXin CONTACT US
CONTACT US










 Tsinghua Holdings works hard for better ecological environment
Tsinghua Holdings works hard for better ecological environment