Novel coronavirus detection kits developed by Tsinghua Holdings subsidiaries obtain CE marking
2020-03-27
A respiratory virus nucleic acid detection kit (isothermal amplification chip method) jointly designed and developed by Tsinghua Holdings’ subsidiary CapitalBio Technology Corporation, Tsinghua University, and West China Hospital of Sichuan University to detect six types of respiratory viruses including the novel coronavirus (2019-nCoV) has obtained CE Marking, which is often referred as the trade passport to Europe for non-EU products.
Another coronavirus nucleic acid detection kit fully developed by ACC Biotech under TusHoldings, a subsidiary of Tsinghua Holdings, has also gained CE Marking approval.
The nucleic acid detection kit products of CapitalBio and ACC Biotech meet the requirements of the In Vitro Diagnostic Medical Devices Directive and may gain access to the EU market, where they will provide an efficient detection tool for the prevention and control of the epidemic.

CapitalBio obtains the CE Technical Documentation Review Report for the Respiratory Virus Nucleic Acid Detection Kit (Isothermal Amplification Chip Method).
Led by Cheng Jing, academician of the Chinese Academy of Engineering, Professor of the School of Medicine, Tsinghua University, and President of CapitalBio Group, CapitalBio has taken quick actions in the fight against the COVID-19, applying its rich experience and technical capacities in the precise diagnosis of pathogenic microorganisms.
The company took just a week to develop the world's only respiratory virus nucleic acid detection kit (isothermal amplification chip method) that can detect six types of respiratory viruses including the novel coronavirus at one time within 1.5 hours. The detection kit has been approved by China’s National Medical Products Administration to be used clinically as a novel coronavirus emergency medical device.
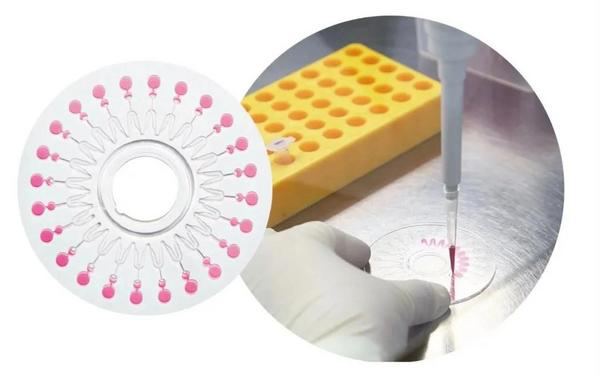
CapitalBio develops a respiratory virus nucleic acid detection kit (isothermal amplification chip method) that can detect six types of respiratory viruses including the novel coronavirus at one time.
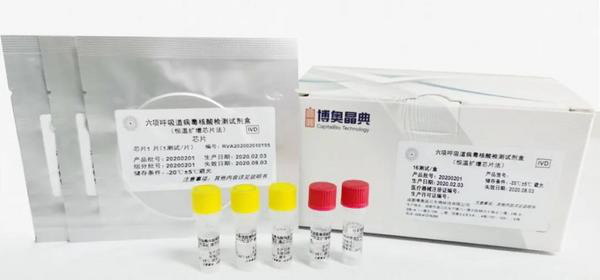
CapitalBio Respiratory Virus Nucleic Acid Detection Kit
CapitalBio donated the first batch of 12,000 detection kits to Wuhan City, Hubei Province to help front line hospitals concentrate short-handed medical resources in the treatment of confirmed novel coronavirus pneumonia patients.
Most of the novel coronavirus nucleic acid detection products can only identify novel coronavirus and distinguish between healthy people and those infected with it. Other tests are needed to further differentiate between COVID-19 patients and flu patients.
CapitalBio’s respiratory virus nucleic acid detection kit and its isothermal nucleic acid amplification microfluidic chip analyzer can solve all the problems simultaneously, which improves diagnosis efficiency and makes treatment available more quickly.
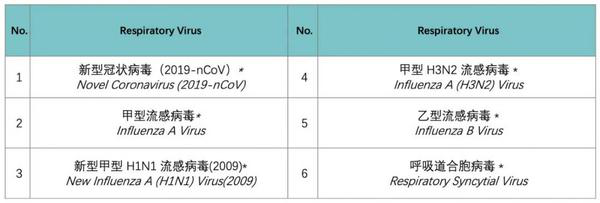
CapitalBio’s respiratory virus nucleic acid detection kit can test novel coronavirus (2019-nCov), Influenza A Virus, New Influenza A (H1N1) Virus (2009), Influenza A (H3N2) Virus, Influenza B Virus, and Respiratory Syncytial Virus.
In addition, the detection kit products have been donated to countries and regions with serious COVID-19 epidemic outbreaks such as Italy and Georgia. With CE certification, the detection kit will help more countries battle the epidemic.

ACC Biotech obtains the CE Technical File Review Report for the Novel Coronavirus SARS-CoV-2 Nucleic Acid detection Kit (Fluorescent PCR).
The Novel Coronavirus SARS-CoV-2 Nucleic Acid detection kit product independently developed by ACC Biotech uses the Biomark® RT-PCR technology featuring high sensitivity, specificity and accuracy. It only takes 1.5 hours to determine the ORF1ab and N gene of novel coronavirus, detecting the virus accurately, efficiently, and conveniently.
The new detection kit for COVID-19 testing has a unique advantage over other methods in detecting asymptomatic or latent virus carriers, and can provide users with reliable, stable and accurate clinical testing support.

 Facebook
Facebook WeiXin
WeiXin CONTACT US
CONTACT US










 Tsinghua Holdings works hard for better ecological environment
Tsinghua Holdings works hard for better ecological environment