CapitalBio’s Isothermal Microfludic Chip Analyzer gains CE certification
2020-04-28
The Isothermal Microfludic Chip Analyzer, a new type of nucleic acid analyzer product independently developed by CapitalBio Technology Corporation, a subsidiary of CapitalBio Corporation under Tsinghua Holdings, gained CE certification on April 27, 2020.
All of CapitalBio’s fast respiratory virus and respiratory pathogens nucleic acid detection products, including detection kits and supporting instruments, have both CE and medical devices certification. They will serve as powerful and efficient detection tools for the worldwide prevention and control of the novel coronavirus, or COVID-19.

CapitalBio’s Isothermal Microfludic Chip Analyzer gains CE-VID certification.

CapitalBio RTisochip™-W High-throughput Isothermal Microfludic Chip Analyzer
The RTisochip™-W High-throughput Isothermal Microfludic Chip Analyzer represents a new generation of multi-sample high-throughput analysis and detection instruments and has proved efficient in widespread prevention and control of COVID-19. It has four individual modules that work independently and simultaneously, and could be flexibly combined for higher capacity when needed. Each module can process multiple samples quickly and simultaneously.
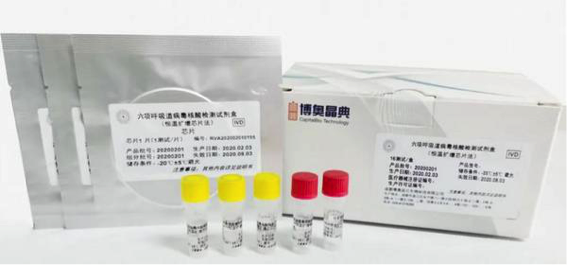
CapitalBio Respiratory Virus Nucleic Acid Detection Kit (isothermal amplification chip method)
The company’s respiratory virus nucleic acid detection kit (isothermal amplification chip method), which works with the RTisochip™-W High-throughput Isothermal Microfludic Chip Analyzer, obtained the CE Marking earlier in March.
The detection kit is able to detect six types of respiratory viruses including the novel coronavirus (2019-nCoV) at one time within 1.5 hours with a patient's nasal and pharyngeal swabs and other secretion samples.

Employees of CapitalBio work in frontline hospitals in Wuhan city, Hubei province, with CapitalBio’s detection kits and instruments to fight against the COVID-19.
In late February, CapitalBio donated 12,000 detection kits to Wuhan City, Hubei Province to help frontline hospitals concentrate short-handed medical resources in the treatment of confirmed novel coronavirus pneumonia patients.
Most of the novel coronavirus nucleic acid detection products in the market can only identify novel coronavirus and distinguish between healthy and infected people. Other tests are needed to further differentiate between COVID-19 patients and flu patients.
CapitalBio’s respiratory virus nucleic acid detection kit and its isothermal nucleic acid amplification microfluidic chip analyzer can solve all the problems simultaneously, which improves diagnosis efficiency and makes treatment available more quickly.
In addition, the CapitalBio Respiratory Pathogens Nucleic Acid Detection Kit is designed for a rapid and accurate detection of the presence of 13 respiratory pathogens. It employs isothermal amplification technology and works with the CapitalBio RTisochip Isothermal Nucleic Acid Amplification Microfluidic Chip Analyzer. The kit and chip analyzer gained CE certification in 2016 and 2017.
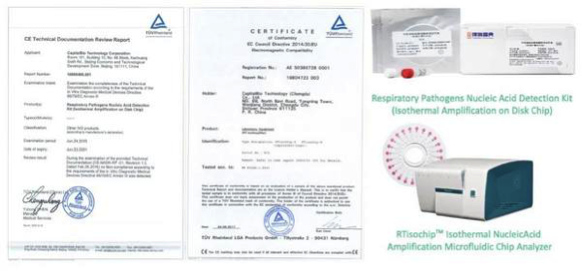
CapitalBio RTisochip Isothermal Nucleic Acid Amplification Microfluidic Chip Analyzer
The CapitalBio’s series of respiratory pathogens and respiratory virus nucleic acid detection products have all obtained CE certifications and those for medical devices. They can be used for rapid differential diagnosis of patients with fever and for the treatment of severe and critically ill patients.
The respiratory virus nucleic acid detection kit that can detect six types of respiratory viruses at one time has already been donated to epidemic impacted countries and regions such as Italy and Georgia.
As the company’s Isothermal Microfludic Chip Analyzer has obtained CE certification it will help more countries progress more easily in the fight against COVID-19.

 Facebook
Facebook WeiXin
WeiXin CONTACT US
CONTACT US










 Tsinghua Holdings works hard for better ecological environment
Tsinghua Holdings works hard for better ecological environment