CapitalBio Technology awarded for its CSR efforts
2020-12-14
Tsinghua Holdings' subsidiary CapitalBio Biochemical Technology Co won a corporate social responsibility (CSR) pioneer award at this year's China Economic Media Summit in Beijing on Dec 4.

CapitalBio Technology was awarded as a CSR pioneer by China Times and the China Economic Media Association. [Photo/chinatimes.net.cn]
The company has not only developed various rapid detection chip systems and mobile in-vehicle labs for COVID-19 nucleic acid testing, but also cooperated with many hospitals to build P2 labs to improve China's testing capabilities and efficiency.
It undertook the task of nucleic acid testing during the outbreak in Beijing, Dalian, Qingdao, Shanghai and the Xinjiang Uygur autonomous region, contributing scientific and technological strength to the country's epidemic prevention.
"CapitalBio has always paid great attention to public welfare," said Wang Guoqing, vice president of the firm.

Wang Guoqing, vice president of CapitalBio, delivers a speech at the China Economic Media Summit in Beijing on Dec 4. [Photo/chinatimes.net.cn]
In response to the call of the anti-epidemic frontline at the beginning of the year, the enterprise rolled out a nucleic acid detection kit which was approved by the State Food and Drug Administration on Feb 22.
This kit can detect six respiratory viruses, including the novel coronavirus, within 1.5 hours. In this way, it is quick to distinguish between flu victims and those infected by COVID-19.
At the same time, CapitalBio donated 12,000 chip kits to Wuhan as quickly as possible.
"In order to use this detection technology in the frontline better and faster, we sent a team to the hospitals for training on how to use it on site," Wang said.
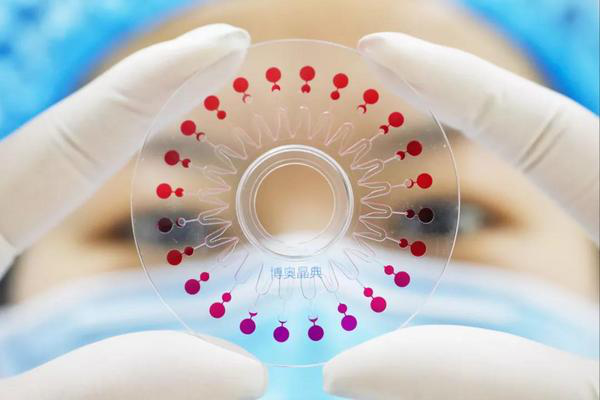
A rendering of the respiratory poly-virus nucleic acid detection chip system [Photo/thholding.com]
Notably, the life science provider also released a pathogen detection chip. The two chips can be used in combination for the greatest effect on anti-COVID-19 work.
"We have known from the test results that most severe patients have mixed infections caused not only by the novel coronavirus but also other bacteria, " Wang added, "It's important to quickly identify their infections and then to adopt targeted treatment."
To satisfy regular anti-epidemic needs, CapitalBio developed a van-based mobile lab that includes oral throat swab sampling robots, rapid virus inactivation instruments and a 5G direct reporting system.
The fully-automatic and portable detection system integrates all steps into an enclosed room and means the entire process can be carried out unaffected by the site environment or the technical skills available.
In this context, the new nucleic acid test can be completed within 45 minutes, more than three times faster than conventional PCR tests. The product is expected to meet the needs of flexible inspections for entry and exit at ports and of emergency medical services during epidemic prevention work.

Design sketch of the integrated nucleic acid detection chip system [Photo/thholding.com]

Design sketch of the mobile lab for nucleic acid detection [Photo/thholding.com]
To date, the company has offered nucleic acid testing services to more than 5 million people across the country.
Nearly 20 of its branch third-party inspection chains have obtained the qualification for nucleic acid testing. They played important roles in screening of key population groups and resumption of business production and academic studies.
In November, it took only two hours for the enterprise to send a medical team to assist Tianjin. "Wherever there is an epidemic, our team will go to support local nucleic acid testing," Wang said.
Sponsored by the China Economic Media Association and China Times, the meeting gathered many senior experts and scholars, as well as editors-in-chief of mainstream financial media and corporate executives.

 Facebook
Facebook WeiXin
WeiXin CONTACT US
CONTACT US










 Tsinghua Holdings works hard for better ecological environment
Tsinghua Holdings works hard for better ecological environment